Ziprasidone
7 Results Found
Page 1 of 1
Ziprasidone API Reference standards and its EP Impurities, USP Impurities, non pharmacopeial Impurities, listed below & also offered custom synthesis for unknown Impurities. If require any contact info@drjcrbio.com, sales@drjcrbio.com
-
Free
Product Name : Ziprasidone HCl Monohydrate; 5-[2-[4-(1,2-Benzisothiazol-3-yl)piperazin-1-yl]ethyl]-6-chloro-1,3-dihydro-2H-indol-2-one hydrochloride monohydrate | 138982-67-9
Code: JCZPS-07 CAS:138982-67-9 Chemical Formula:C21H24Cl2N4O2S Molecular Weight: 467.41 Category: Reference Standards/API Drug Metabolites/EP Impurities/USP Impurities Literature: Application: Ziprasidone HCl Monohydrate ; 5-[2-[4-(1,2-Benzisothiazol-3-yl)piperazin-1-yl]ethyl]-6-chloro-1,3-dihydro-2H-indol-2-one hydrochloride monohydrate is useful as reference Impurity standard. ![Ziprasidone HCl Monohydrate; 5-[2-[4-(1,2-Benzisothiazol-3-yl)piperazin-1-yl]ethyl]-6-chloro-1,3-dihydro-2H-indol-2-one hydrochloride monohydrate | 138982-67-9](https://drjcrbio.com/wp-content/uploads/Ziprasidone-HCl-Monohydrate.png)
-
Free
Product Name : Ziprasidone EP Impurity P; 1,4-Bis(benzo[d]isothiazol-3-yl)piperazine | 223586-82-1
Code: JCZPS-06 CAS: 223586-82-1 Chemical Formula: C18H16N4S2 Molecular Weight: 352.48 Category: Reference Standards/API Drug Metabolites/EP Impurities/USP Impurities Literature: Application: Ziprasidone EP Impurity P; 1,4-Bis(benzo[d]isothiazol-3-yl)piperazine is useful as reference Impurity standard. ![Ziprasidone EP Impurity P; 1,4-Bis(benzo[d]isothiazol-3-yl)piperazine | 223586-82-1](https://drjcrbio.com/wp-content/uploads/Ziprasidone-EP-Impurity-P.png)
-
Free
Product Name : Ziprasidone EP Impurity E; Ziprasidone USP RC D ; 3-(1,2-Benzisothiazol-3-yl)-5-[2-[4-(1,2-benzisothiazol-3-yl)piperazin-1-yl] ethyl]-6-chloro-1,3-dihydro-2H-indol-2-one | 1159977-04-4
Code: JCZPS-05 CAS: 1159977-04-4 Chemical Formula: C28H24ClN5OS2 Molecular Weight: 546.11 Category: Reference Standards/API Drug Metabolites/EP Impurities/USP Impurities Literature: Application: Ziprasidone EP Impurity E; Ziprasidone USP RC D ; 3-(1,2-Benzisothiazol-3-yl)-5-[2-[4-(1,2-benzisothiazol-3-yl)piperazin-1-yl] ethyl]-6-chloro-1,3-dihydro-2H-indol-2-one; CAS:1159977-04-4 is useful as reference Impurity standard. ![Ziprasidone EP Impurity E; Ziprasidone USP RC D ; 3-(1,2-Benzisothiazol-3-yl)-5-[2-[4-(1,2-benzisothiazol-3-yl)piperazin-1-yl] ethyl]-6-chloro-1,3-dihydro-2H-indol-2-one | 1159977-04-4](https://drjcrbio.com/wp-content/uploads/Ziprasidone-EP-Impurity-E.png)
-
Free
Product Name : Ziprasidone EP Impurity D; Ziprasidone Dimer; Ziprasidone USP RC C; 5,5′-bis[2-[4-(1,2-Benzisothiazol-3-yl)piperazin-1-yl]ethyl]-6,6′-dichloro-3-hydroxy-1,1′,3,3′-tetrahydro-2H,2′H-3,3′-biindole-2,2′-dione | 1303996-68-0
Code: JCZPS-04 CAS: 1303996-68-0 Chemical Formula: C42H40Cl2N8O3S2 Molecular Weight: 839.85 Category: Reference Standards/API Drug Metabolites/EP Impurities/USP Impurities Literature: Application: Ziprasidone EP Impurity D; Ziprasidone Dimer; Ziprasidone USP RC C; 5,5′-bis[2-[4-(1,2-Benzisothiazol-3-yl)piperazin-1-yl]ethyl]-6,6′-dichloro-3-hydroxy-1,1′,3,3′-tetrahydro-2H,2′H-3,3′-biindole-2,2′-dione; CAS: 1303996-68-0is useful as reference Impurity standard. ![Ziprasidone EP Impurity D; Ziprasidone Dimer; Ziprasidone USP RC C; 5,5′-bis[2-[4-(1,2-Benzisothiazol-3-yl)piperazin-1-yl]ethyl]-6,6′-dichloro-3-hydroxy-1,1′,3,3′-tetrahydro-2H,2′H-3,3′-biindole-2,2′-dione | 1303996-68-0](https://drjcrbio.com/wp-content/uploads/Ziprasidone-EP-Impurity-D.png)
-
Free
Product Name : Ziprasidone EP Impurity C; Ziprasidone Open-Ring Impurity; Ziprasidone Amino Acid Sodium Salt ; 2-[2-Amino-5-[2-[4-(1,2-benzisothiazol-3-yl)piperazin-1-yl]ethyl]-4-chlorophenyl]acetic acid sodium salt | 1159977-64-6
Code: JCZPS-03 CAS: 1159977-64-6 Chemical Formula: C21H22ClN4NaO2S Molecular Weight: 452.93 Category: Reference Standards/API Drug Metabolites/EP Impurities/USP Impurities Literature: Application: Ziprasidone EP Impurity C; Ziprasidone Open-Ring Impurity; Ziprasidone Amino Acid Sodium Salt ; 2-[2-Amino-5-[2-[4-(1,2-benzisothiazol-3-yl)piperazin-1-yl]ethyl]-4-chlorophenyl]acetic acid sodium salt is useful as reference Impurity standard. ![Ziprasidone EP Impurity C; Ziprasidone Open-Ring Impurity; Ziprasidone Amino Acid Sodium Salt ; 2-[2-Amino-5-[2-[4-(1,2-benzisothiazol-3-yl)piperazin-1-yl]ethyl]-4-chlorophenyl]acetic acid sodium salt | 1159977-64-6](https://drjcrbio.com/wp-content/uploads/Ziprasidone-EP-Impurity-C.png)
-
Free
Product Name : Ziprasidone EP Impurity B ; 3-Oxo Ziprasidone ; Ziprasidone USP RC B ; 5-[2-[4-(1,2-Benzisothiazol-3-yl)piperazin-1-yl]ethyl]-6-chloro-1H-indole-2,3-dione | 1159977-56-6
Code: JCZPS-02 CAS: 1159977-56-6 Chemical Formula: C21H19ClN4O2S Molecular Weight: 426.92 Category: Reference Standards/API Drug Metabolites/EP Impurities/USP Impurities Literature: Application: Ziprasidone EP Impurity B ; 3-Oxo Ziprasidone ; Ziprasidone USP RC B ; 5-[2-[4-(1,2-Benzisothiazol-3-yl)piperazin-1-yl]ethyl]-6-chloro-1H-indole-2,3-dione; CAS: 1159977-56-6 is useful as reference Impurity standard. ![Ziprasidone EP Impurity B ; 3-Oxo Ziprasidone ; Ziprasidone USP RC B ; 5-[2-[4-(1,2-Benzisothiazol-3-yl)piperazin-1-yl]ethyl]-6-chloro-1H-indole-2,3-dione | 1159977-56-6](https://drjcrbio.com/wp-content/uploads/Ziprasidone-EP-Impurity-B.png)
-
Free
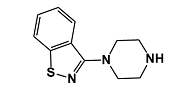
Product Name : Ziprasidone EP Impurity A; Ziprasidone USP RC A; 3-Piperazin-1-yl-1,2-benzisothiazole | 87691-87-0
Code: JCZPS-01 CAS: 87691-87-0 Chemical Formula: C11H13N3S Molecular Weight: 219.31 Category: Reference Standards/API Drug Metabolites/EP Impurities/USP Impurities Literature: Application: Ziprasidone EP Impurity A; Ziprasidone USP RC A; 3-Piperazin-1-yl-1,2-benzisothiazole; CAS: 87691-87-0is useful as reference Impurity standard.