Lovastatin
6 Results Found
Page 1 of 1
Lovastatin API Reference standards and its EP Impurities, USP Impurities, non pharmacopeial Impurities, listed below & also offered custom synthesis for unknown Impurities. If require any contact info@drjcrbio.com, sales@drjcrbio.com
-
Free
Product Name : Lovastatin Impurity F
Code: JCLOV-06 CAS: Chemical Formula: C24H34O5 Molecular Weight: 402.5 Category: Reference Standards/API Drug Metabolites/EP Impurities/USP Impurities Literature: Application: Lovastatin Impurity F is useful as reference Impurity standard 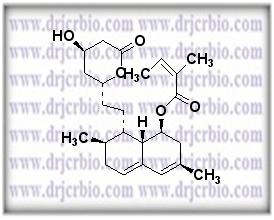
-
Free
Product Name : Lovastatin Dimer
Code: JCLOV-05 CAS: Chemical Formula: C48H72O10 Molecular Weight: 809.1 Category: Reference Standards/API Drug Metabolites/EP Impurities/USP Impurities Literature: Application: Lovastatin Dimer is useful as reference Impurity standard 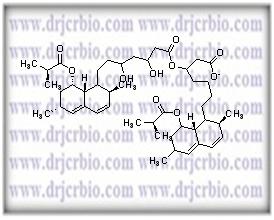
-
Free
Product Name : Hydroxy Lovastatin
Code: JCLOV-04 CAS: Chemical Formula: C24H36O7 Molecular Weight: Category: Reference Standards/API Drug Metabolites/EP Impurities/USP Impurities Literature: Application: Hydroxy Lovastatin is useful as reference Impurity standard 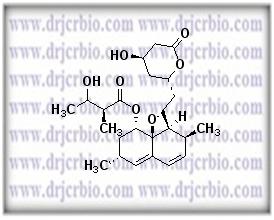
-
Free
Product Name : Lovastatin
Code: JCLOV-03 CAS: Chemical Formula: C24H36O5 Molecular Weight: Category: Reference Standards/API Drug Metabolites/EP Impurities/USP Impurities Literature: Application:Lovastatin is useful as reference Impurity standard 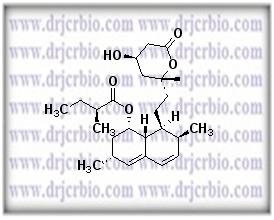
-
Free
Product Name : Des(2-methylbutyrate) lovastatin hydroxy acid sodium salt
Code: JCLOV-02 CAS: Chemical Formula: C19H29O5.Na Molecular Weight: Category: Reference Standards/API Drug Metabolites/EP Impurities/USP Impurities Literature: Application: Des(2-methylbutyrate) lovastatin hydroxy acid sodium salt is useful as reference Impurity standard 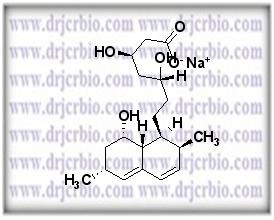
-
Free
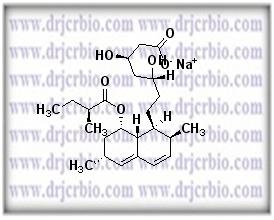
Product Name : Lovastatin hydroxy acid sodium salt
Code: JCLOV-01 CAS: Chemical Formula: C24H37O6.Na Molecular Weight: Category: Reference Standards/API Drug Metabolites/EP Impurities/USP Impurities Literature: Application: Lovastatin hydroxy acid sodium salt is useful as reference Impurity standard