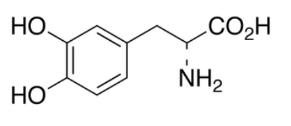
Levodopa
4 Results Found
Page 1 of 1
Levodopa API Reference standards and its EP Impurities, USP Impurities, non pharmacopeial Impurities, listed below & also offered custom synthesis for unknown Impurities. If require any contact info@drjcrbio.com, sales@drjcrbio.com
-
Free
Product Name : Levodopa-3-acetyl L-tyrosine impurity ;(S)-3-(3-acetyl-4-hydroxyphenyl)-2-aminopropanoic acid hydrochloride |32404-28-7
Code: JCLVD-04 CAS: 32404-28-7 Chemical Formula: C11H14ClNO4 Molecular Weight:259.69 Category: Levodopa Reference Standards/Levodopa API Drug Metabolites/Levodopa EP Impurities/Levodopa USP Impurities Literature: Application: Levodopa-3-acetyl L-tyrosine impurity ;(S)-3-(3-acetyl-4-hydroxyphenyl)-2-aminopropanoic acid hydrochloride |32404-28-7 is useful as reference Impurity standard. 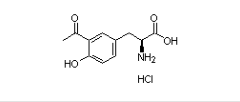
-
Free
Product Name : Levodopa EP Impurity A / Levodopa Related Compound A ;(2S)-2-Amino-3-(2,4,5-trihydroxyphenyl)propanoic acid;3-(3,4,6-Trihydroxyphenyl)-alanine ;2-Hydroxy Levodopa |27244-64-0
Code: JCLVD-03 CAS: 27244-64-0 Chemical Formula:C9H11NO5 Molecular Weight:213.19 Category: Reference Standards/API Drug Metabolites/EP Impurities/USP Impurities Literature: Application: Levodopa EP Impurity A / Levodopa Related Compound A ;(2S)-2-Amino-3-(2,4,5-trihydroxyphenyl)propanoic acid;3-(3,4,6-Trihydroxyphenyl)-alanine ;2-Hydroxy Levodopa |27244-64-0is useful as reference Impurity standard. 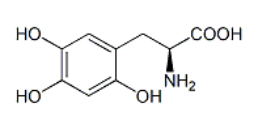
-
Free
Product Name : LEVODOPA RELATED COMPOUND B ;Levodopa EP Impurity C;Levodopa EP Impurity C;Levodopa USP Related Compound B;(2RS)-2-Amino-3-(4-hydroxy-3-methoxyphenyl)propanoic acid;3-Methyl-DL-Dopa;3-Methoxy-DL-tyrosine ; |7636-26-2
Code: JCLVD-02 CAS: 7636-26-2 Chemical Formula: C10H13NO4. Molecular Weight: 211.21 Category: Reference Standards/API Drug Metabolites/EP Impurities/USP Impurities Literature: Application: LEVODOPA RELATED COMPOUND B ;Levodopa EP Impurity C;Levodopa EP Impurity C;Levodopa USP Related Compound B;(2RS)-2-Amino-3-(4-hydroxy-3-methoxyphenyl)propanoic acid;3-Methyl-DL-Dopa;3-Methoxy-DL-tyrosine ; |7636-26-2 is useful as reference Impurity standard. 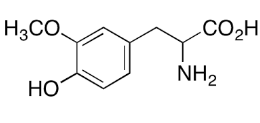
-
Free
Product Name : Levodopa EP impurity D (D-dopa) ;(2R)-2-amino-3-(3,4-Dihydroxyphenyl)propanoic acid | 5796-17-8
Code: JCLVD-01 CAS: 5796-17-8 Chemical Formula: C9H11NO4 Molecular Weight: 197.19 Category: Reference Standards/API Drug Metabolites/EP Impurities/USP Impurities Literature: Application: Levodopa EP impurity D (D-dopa) ;(2R)-2-amino-3-(3,4-Dihydroxyphenyl)propanoic acid | 5796-17-8 is useful as reference Impurity standard.