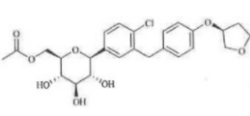
Empagliflozin MAE Impurity
1 Results Found
Page 1 of 1
Dapagliflozin API Reference standards and its EP Impurities, USP Impurities, non pharmacopeial Impurities, listed below & also offered custom synthesis for unknown Impurities. If require any contact info@drjcrbio.com, sales@drjcrbio.com
-
Free
Product Name : Empagliflozin MAE Impurity ;((2R,3S,4R,5R,6S)-6-(4-Chloro-3-(4-(((S)-tetrahydrofuran-3-yl)oxybenzyl)phenyl)-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl)methyl acetate
Code: JCDPG-22 CAS: NA Chemical Formula:C25H29ClO8 Molecular Weight:492.95 Category: Reference Standards/API Drug Metabolites/EP Impurities/USP Impurities Literature: Application: Empagliflozin MAE Impurity ;((2R,3S,4R,5R,6S)-6-(4-Chloro-3-(4-(((S)-tetrahydrofuran-3-yl)oxybenzyl)phenyl)-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl)methyl acetateis useful as reference Impurity standard.